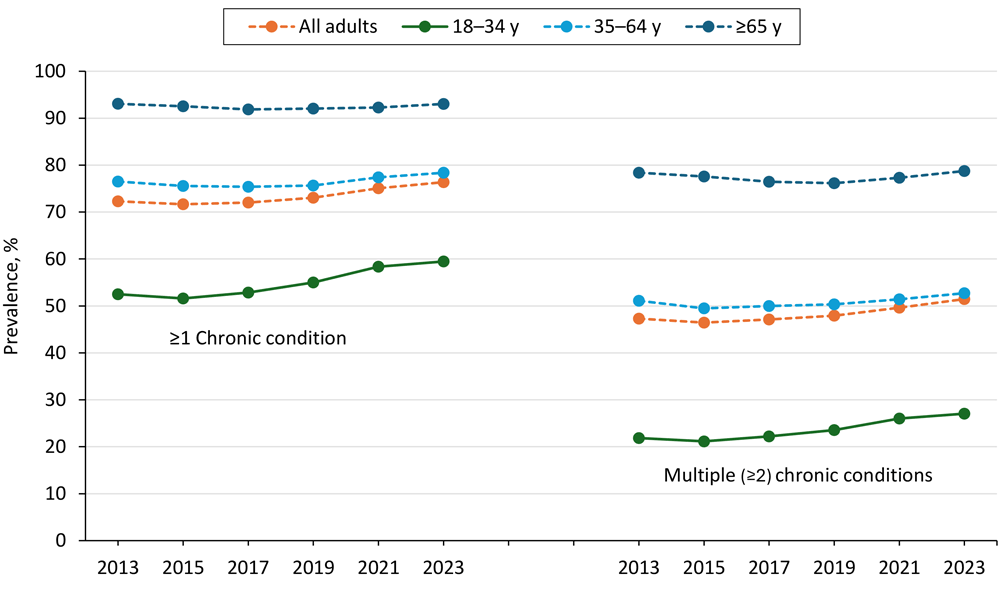The Multimodal Shift: Why Health AI Needs More Than Hospital Data
Author: Peehu Sachdeva, Market Strategy Lead, Axia Medicine
Published by Axia Medicine | November 2025
This is the second article of a series, following "The Genomics Paradox".
In the last decade, the UK Biobank showed what a large, high-quality multimodal dataset can do for research, producing thousands of publications. It also showed how slow and expensive that progress can be. It took a decade for UK Biobank to become the UK Biobank. Few efforts have come close: FinnGen, All of Us, Our Future Health and a handful of others.
In the private sector, Tempus AI’s IPO created a global multi-omics asset, albeit limited to oncology and requiring billions in investment. If every therapeutic area required comparable efforts to make multimodal data accessible, the industry would spend a century waiting for the datasets needed today.
The impact of current initiatives is undeniable; equally undeniable is that we cannot afford to keep waiting for the next breakthrough. And as we explored in The Genomic Paradox, this lag in discovery is not technical, it is structural. If slogans like "health data is commoditized" were true, how hard could it be to make diverse, multimodal data widely available?
Today's data collection systems are simply unable to fix the shortage of multimodal and multiomics data: they were built for institutions, not for individuals.
And in Precision Medicine, data is always singular.
From Short Reads to Multimodal Intelligence
The Genomics Paradox highlighted that sequencing is abundant but access remains scarce. Each new sensor, wearable, and assay generates insight into human biology, yet most measurements end up in silos behind institutional or commercial walls.
No slogan can bridge these gaps. Even the most advanced model cannot learn from data it cannot see, and synthetic data still depends on real patient data to exist. To reach its potential, AI must be native to multimodality, integrating genomics, proteomics, imaging, clinical notes and behavioural data from inception, not as an add-on.
When GSK, Regeneron, and UK Biobank combined 500,000 exomes with longitudinal health records, the result unlocked discoveries unreachable by either modality alone. That partnership proved that the availability of multimodal data can drive progress across entire industries, globally.
The Data We Ignore in Everyday Health
Consider a young diabetes patient. Their record captures pristine billing codes and lab results but says nothing about the food desert they live in or the stress of working two jobs. Roughly 3 in 4 U.S. adults now live with one or more chronic conditions (Figure 1), many of which are influenced by nutrition, genetics and environment. Yet the most advanced health systems still operate on fragments of information.
Even beyond omics, most of our health data lives outside the clinic: in behaviour, in our daily routines and environmental exposure. That is the inevitable truth: care delivery still relies on a fraction of the patient’s story. Industry keeps optimising for what is easy to measure, missing what truly determines outcomes.
When AI systems learn from incomplete context, their limitations go far beyond accuracy metrics. Without longitudinal, multimodal insight, predictions risk being misapplied or misunderstood. Addressing this challenge requires strengthening, not bypassing, the structures that ensure data is used responsibly.
Figure 1. U.S. trends in single and multiple chronic conditions (2013-2023). Most age groups show rising prevalence over time. Solid lines mark meaningful changes; dashed lines indicate no significant trend.
Source: Centers for Disease Control and Prevention. Trends in Multiple Chronic Conditions Among US Adults, By Life Stage, Behavioral Risk Factor Surveillance System, 2013–2023.
Native Multimodal AI and Data Liquidity
Most platforms today are retrofitted for multiple data types, losing the relationships that make multimodal data valuable. Native architectures preserve those connections in real time, allowing AI to detect how genetics, behaviour, environment and clinical care interact to shape health outcomes.
Achieving this vision depends on collaboration. Clinics and health systems are uniquely positioned to provide longitudinal continuity, clinical interpretation, and ethical oversight. Patients contribute essential data and consent. Researchers bring analytical expertise. Progress emerges when these roles are aligned within governed frameworks.
Rather than focusing solely on where data originates, the more important question is how data moves. Safe, purpose-specific data flow requires transparency, traceability, and shared value. When access is governed and consent-driven, data can support research while respecting patient expectations and clinical responsibility.
That is how multimodal AI compounds in value and reliability for the long term, starting with individual patients and, as Precision Public Health details, encompassing the wider population.
Participatory Medicine: Health AI's First Job
The promise of multimodal AI is not only smarter prediction, but more meaningful participation. When patients are supported by trusted care providers and clear consent frameworks, engagement can be sustained over time. This continuity is what enables diversity, reliability, and learning at scale.
Precision Medicine has always aimed to treat patients as individuals. Achieving that goal requires infrastructure that reflects the full picture of health, while remaining grounded in clinical stewardship and ethical research practice. The future of multimodal AI will shaped by the quality of partnerships that make data usable, trustworthy, and relevant to care.
At AXIA, we believe progress depends on enabling patients and clinics to participate together in ethical, consent-driven research collaboration. By supporting governed access to multimodal and longitudinal data, we aim to help translate innovation into lasting value for patients, providers, and the broader healthcare ecosystem
References
Ardic, N. and Dinc, R. (2025). Emerging trends in multi-modal artificial intelligence for clinical decision support: A narrative review. PubMed, 31(3), p.14604582251366141-14604582251366141. doi:https://doi.org/10.1177/14604582251366141.
Bijan Mossadeghi, Caixeta, R., Ondarsuhu, D., Luciani, S., Hambleton, I.R. and Hennis, A.J.M. (2023). Multimorbidity and social determinants of health in the US prior to the COVID-19 pandemic and implications for health outcomes: a cross-sectional analysis based on NHANES 2017–2018. BMC Public Health, 23(1). doi:https://doi.org/10.1186/s12889-023-15768-8.
Chustecki, M. (2024). Benefits and Risks of AI in Health Care: Narrative Review. Interactive Journal of Medical Research, [online] 13(e53616). doi:https://doi.org/10.2196/53616.
El Arab, R.A., Abu-Mahfouz, M.S., Abuadas, F.H., Alzghoul, H., Almari, M., Ghannam, A. and Seweid, M.M. (2025). Bridging the Gap: From AI Success in Clinical Trials to Real-World Healthcare Implementation—A Narrative Review. Healthcare, 13(7), p.701. doi:https://doi.org/10.3390/healthcare13070701.
Hao, Y., Cheng, C., Li, J., Li, H., Di, X., Zeng, X., Jin, S., Han, X., Liu, C., Wang, Q., Luo, B., Zeng, X. and Li, K. (2025). Multimodal Integration in Healthcare: Development with Applications in Disease Management (Preprint). Journal of Medical Internet Research. [online] doi:https://doi.org/10.2196/76557.
Poon, H. (2023). Multimodal Generative AI for Precision Health. NJEM AI. [online] doi:https://ai.nejm.org/doi/10.1056/AI-S2300233.
Schouten, D., Nicoletti, G., Dille, B., Chia, C., Vendittelli, P., Schuurmans, M., Litjens, G. and Khalili, N. (2025). Navigating the landscape of multimodal AI in medicine: A scoping review on technical challenges and clinical applications. Medical image analysis, [online] 105, p.103621. doi:https://doi.org/10.1016/j.media.2025.103621.
Shaik, T., Tao, X., Li, L., Xie, H. and Velásquez, J.D. (2024). A survey of multimodal information fusion for smart healthcare: Mapping the journey from data to wisdom. Information Fusion, [online] 102, p.102040. doi:https://doi.org/10.1016/j.inffus.2023.102040.
Simon, B., Ozyoruk, K., Gelikman, D., Harmon, S. and Türkbey, B. (2024). The future of multimodal artificial intelligence models for integrating imaging and clinical metadata: A narrative review. Diagn Interv Radiol. [online] doi:https://doi.org/10.4274/dir.2024.242631.
Sudlow, C., Gallacher, J., Allen, N., Beral, V., Burton, P., Danesh, J., Downey, P., Elliott, P., Green, J., Landray, M., Liu, B., Matthews, P., Ong, G., Pell, J., Silman, A., Young, A., Sprosen, T., Peakman, T. and Collins, R. (2015). UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Medicine, 12(3), p.e1001779. doi:https://doi.org/10.1371/journal.pmed.1001779.
Van Hout, C.V., Tachmazidou, I., Backman, J.D., Hoffman, J.D., Liu, D., Pandey, A.K., Gonzaga-Jauregui, C., Khalid, S., Ye, B., Banerjee, N., Li, A.H., O’Dushlaine, C., Marcketta, A., Staples, J., Schurmann, C., Hawes, A., Maxwell, E., Barnard, L., Lopez, A. and Penn, J. (2020). Exome sequencing and characterization of 49,960 individuals in the UK Biobank. Nature, [online] 586(7831), pp.749–756. doi:https://doi.org/10.1038/s41586-020-2853-0.
Watson, K.B., Wiltz, J.L., Nhim, K., Kaufmann, R.B., Thomas, C.W. and Greenlund, K.J. (2025). Trends in Multiple Chronic Conditions Among US Adults, By Life Stage, Behavioral Risk Factor Surveillance System, 2013–2023. Preventing Chronic Disease, [online] 22.